Glycoscience Products & Services

 Sodium Alginate CAS 9005-38-3
Sodium Alginate CAS 9005-38-3Sodium salt of a polysaccharide obtained from the brown seaweeds (eg Laminaria hyperborea, Fucus vesiculosus, Ascophyllum nodosum).
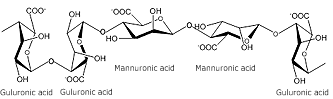
The chemical structure consists of blocks of (1,4) linked-β-D-polymannuronic acid (poly M), (1,4) linked-α-L-polyguluronic acid (poly G) and alternating blocks of the two uronic acids (poly MG).
The main use for alginate is in textile printing where it is used in the printing of cottons with reactive dyes as a thickener. It is used as a thickener and gelling agent in the food industry. Recently, it has been shown that ternary mixtures of Konjac glucomannan, Xanthan gum and Sodium alginate can form a non-covalently linked complex which exhibits enhanced rheological properties of value in, for example, functional foods (Ali Saber Abdelhameed, Shirley Ang, Gordon A. Morris, Ian Smith, Chris Lawson, Roland Gahler, Simon Wood and Stephen E. Harding, An Analytical Ultracentrifuge Study on Ternary Mixtures of Konjac Glucomannan supplemented with Sodium Alginate and Xanthan Gum, Carbohydrate Polymers, 81, (2010), 145-148. Stephen E. Harding, Ian H. Smith, Christopher J Lawson, Roland Gahler, & Simon Wood, Studies on Macromolecular Interactions in Ternary Mixtures of Konjac Glucomannan, Xanthan Gum and Sodium Alginate, Carbohydrate Polymers, 83(2), (2011), 317-328).
Alginates form strong gels with divalent metal cations and the egg box model has been used to describe this form of gelation (see below).