Methylcellulose is a water-soluble polymer used as a binder or thickener in pharmaceutical, food, and ceramic processing applications. The methylation of a number of the polysaccharide hydroxyl groups causes hydrogen bond disruption and methylcellulose becomes water-soluble.
Methylcellulose has an unusual lower critical solution temperature (LCST) between 40 °C and 50 °C. At temperatures below the LCST it is readily soluble in water; above the LCST it is not soluble, which has a paradoxical effect that heating a saturated solution of methylcellulose will turn it solid, because methylcellulose will precipitate out. The temperature at which this occurs depends on DS-value, with higher DS-values giving lower solubility and lower precipitation temperatures because the polar hydroxyl groups are masked.



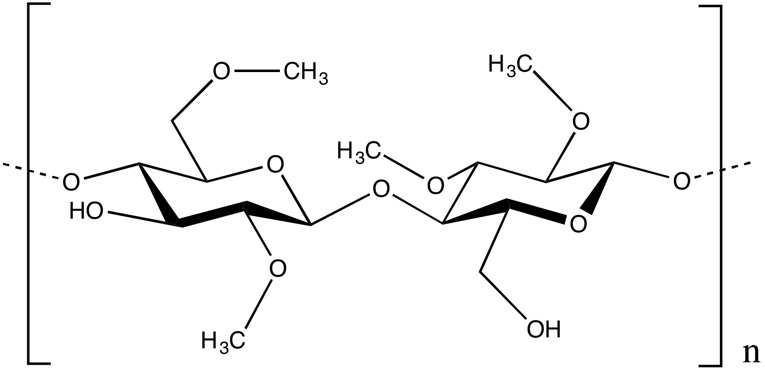 Methyl cellulose CAS 9004-67-5
Methyl cellulose CAS 9004-67-5